Running Multiple Chains (Sequentially or in Parallel) in StochTree
Source:vignettes/MultiChain.Rmd
MultiChain.RmdMotivation
Mixing of an MCMC sampler is a perennial concern for complex Bayesian models. BART and BCF are no exception. On common way to address such concerns is to run multiple independent “chains” of an MCMC sampler, so that if each chain gets stuck in a different region of the posterior, their combined samples attain better coverage of the full posterior.
This idea works with the classic “root-initialized” MCMC sampler of Chipman, George, and McCulloch (2010), but a key insight of He and Hahn (2023) and Krantsevich, He, and Hahn (2023) is that the GFR algorithm may be used to warm-start initialize multiple chains of the BART / BCF MCMC sampler.
Operationally, the above two approaches have the same implementation
(setting num_gfr > 0 if warm-start initialization is
desired), so this vignette will demonstrate how to run a multi-chain
sampler sequentially or in parallel.
To begin, load stochtree and other necessary
packages
Demo 1: Supervised Learning
Data Simulation
Simulate a simple partitioned linear model
# Generate the data
set.seed(1111)
n <- 500
p_x <- 10
p_w <- 1
snr <- 3
X <- matrix(runif(n * p_x), ncol = p_x)
leaf_basis <- matrix(runif(n * p_w), ncol = p_w)
f_XW <- (((0 <= X[, 1]) & (0.25 > X[, 1])) *
(-7.5 * leaf_basis[, 1]) +
((0.25 <= X[, 1]) & (0.5 > X[, 1])) * (-2.5 * leaf_basis[, 1]) +
((0.5 <= X[, 1]) & (0.75 > X[, 1])) * (2.5 * leaf_basis[, 1]) +
((0.75 <= X[, 1]) & (1 > X[, 1])) * (7.5 * leaf_basis[, 1]))
noise_sd <- sd(f_XW) / snr
y <- f_XW + rnorm(n, 0, 1) * noise_sd
# Split data into test and train sets
test_set_pct <- 0.2
n_test <- round(test_set_pct * n)
n_train <- n - n_test
test_inds <- sort(sample(1:n, n_test, replace = FALSE))
train_inds <- (1:n)[!((1:n) %in% test_inds)]
X_test <- X[test_inds, ]
X_train <- X[train_inds, ]
leaf_basis_test <- leaf_basis[test_inds, ]
leaf_basis_train <- leaf_basis[train_inds, ]
y_test <- y[test_inds]
y_train <- y[train_inds]Sampling Multiple Chains Sequentially from Scratch
The simplest way to sample multiple chains of a stochtree model is to
do so “sequentially,” that is, after chain 1 is sampled, chain 2 is
sampled from a different starting state, and similarly for each of the
requested chains. This is supported internally in both the
bart() and bcf() functions, with the
num_chains parameter in the general_params
list.
Define some high-level parameters, including number of chains to run and number of samples per chain. Here we run 4 independent chains with 2000 MCMC iterations, each of which is burned in for 2000 iterations.
num_chains <- 4
num_gfr <- 0
num_burnin <- 1000
num_mcmc <- 2000Run the sampler
bart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
num_gfr = num_gfr,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = list(num_chains = num_chains)
)Now we have a bartmodel object with
num_chains * num_mcmc samples stored internally. These
samples are arranged sequentially, with the first num_mcmc
samples corresponding to chain 1, the next num_mcmc samples
to chain 2, etc…
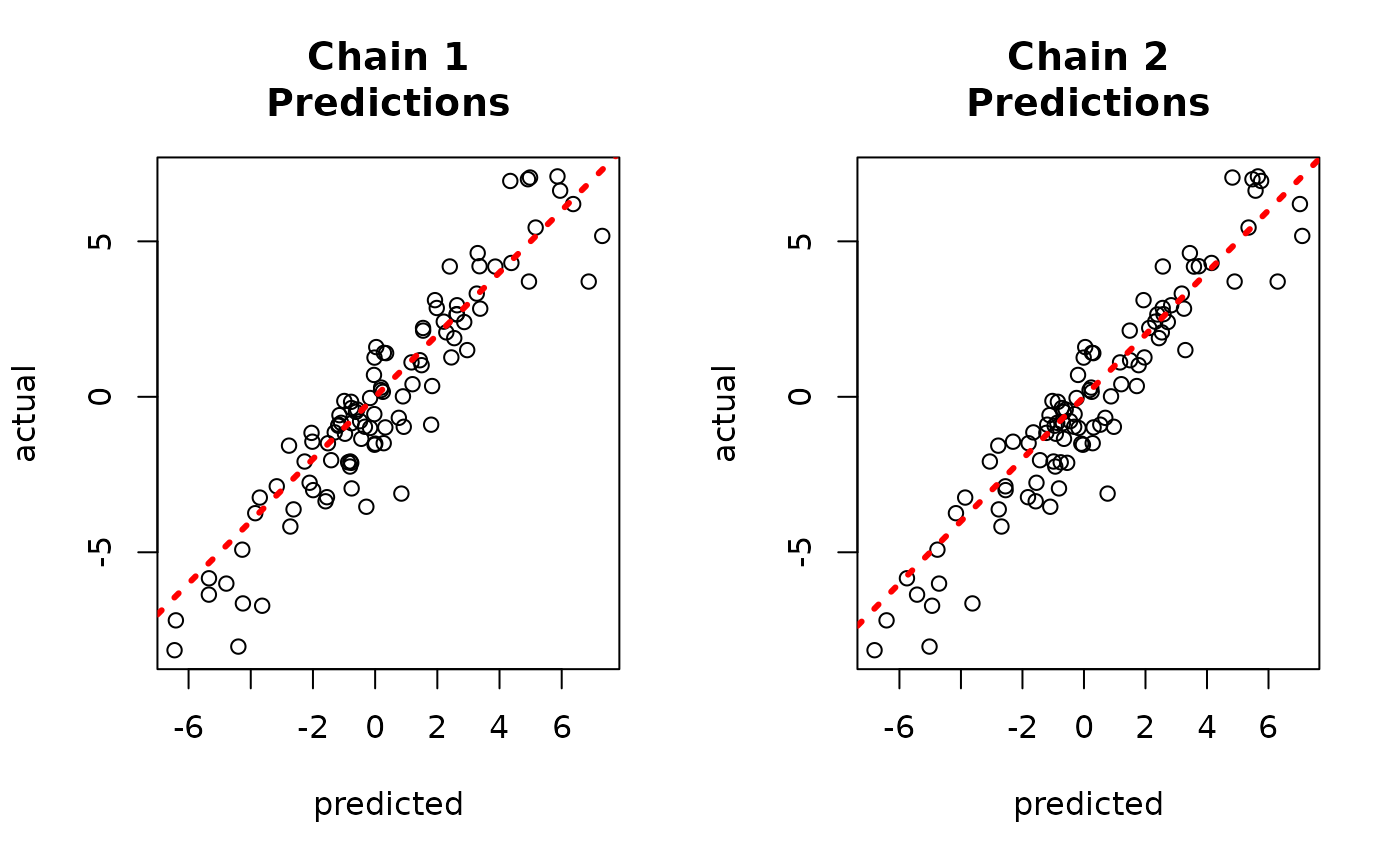
Since each chain is a set of samples of the same model, we can analyze the samples collectively, for example, by looking at out-of-sample predictions.
y_hat_test <- predict(
bart_model,
X = X_test,
leaf_basis = leaf_basis_test,
type = "mean",
terms = "y_hat"
)
plot(y_hat_test, y_test, xlab = "Predicted", ylab = "Actual")
abline(0, 1, col = "red", lty = 3, lwd = 3)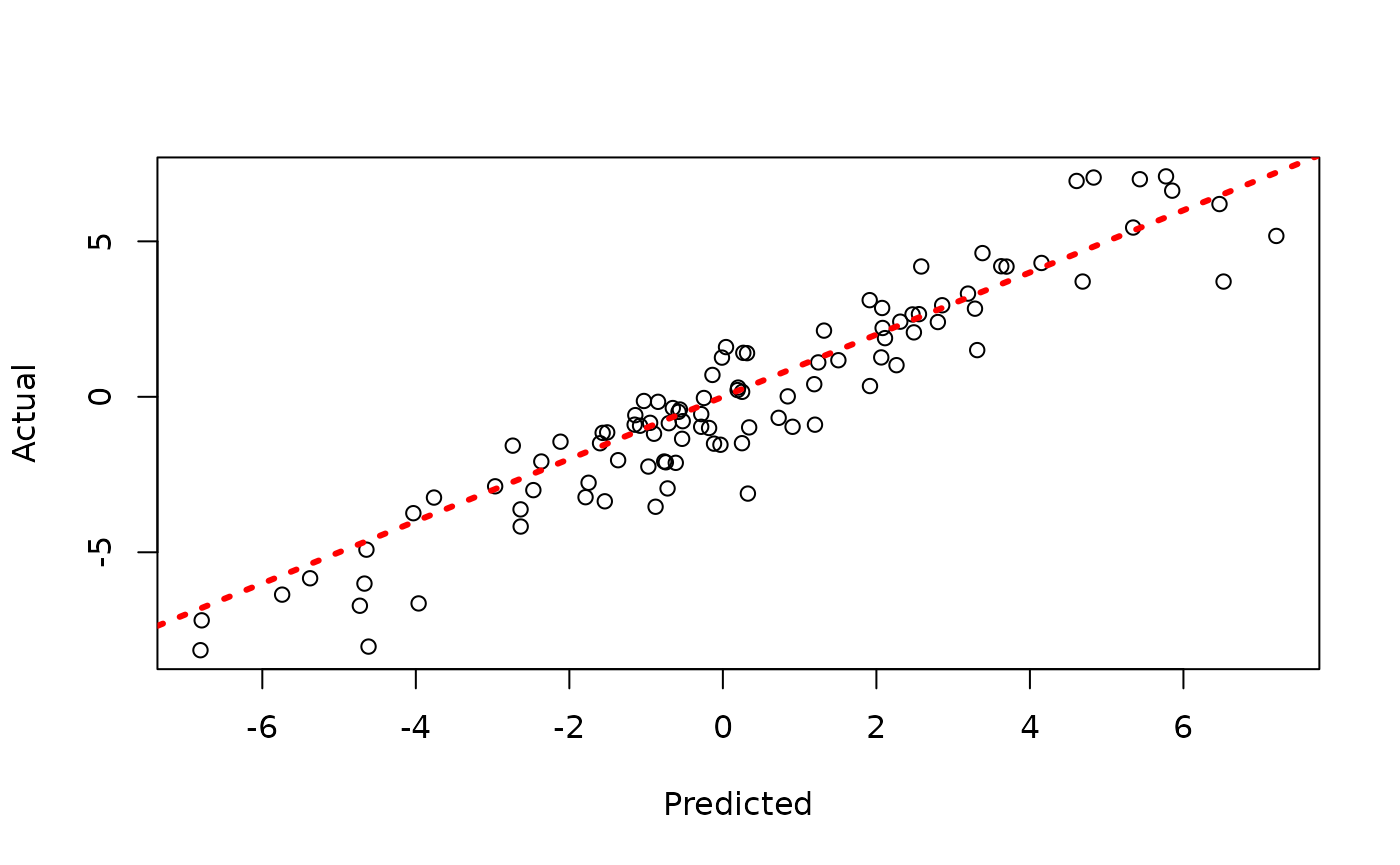
Now, suppose we want to analyze each of the chains separately to assess mixing / convergence.
We can use our knowledge of the internal arrangement of the chain
samples to construct a an mcmc.list in the
coda package, from which we can perform various
diagnostics.
sigma2_coda_list <- coda::as.mcmc.list(lapply(
1:num_chains,
function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(bart_model$sigma2_global_samples[inds_start:inds_end])
}
))
traceplot(sigma2_coda_list, ylab = expression(sigma^2))
abline(h = noise_sd^2, col = "black", lty = 3, lwd = 3)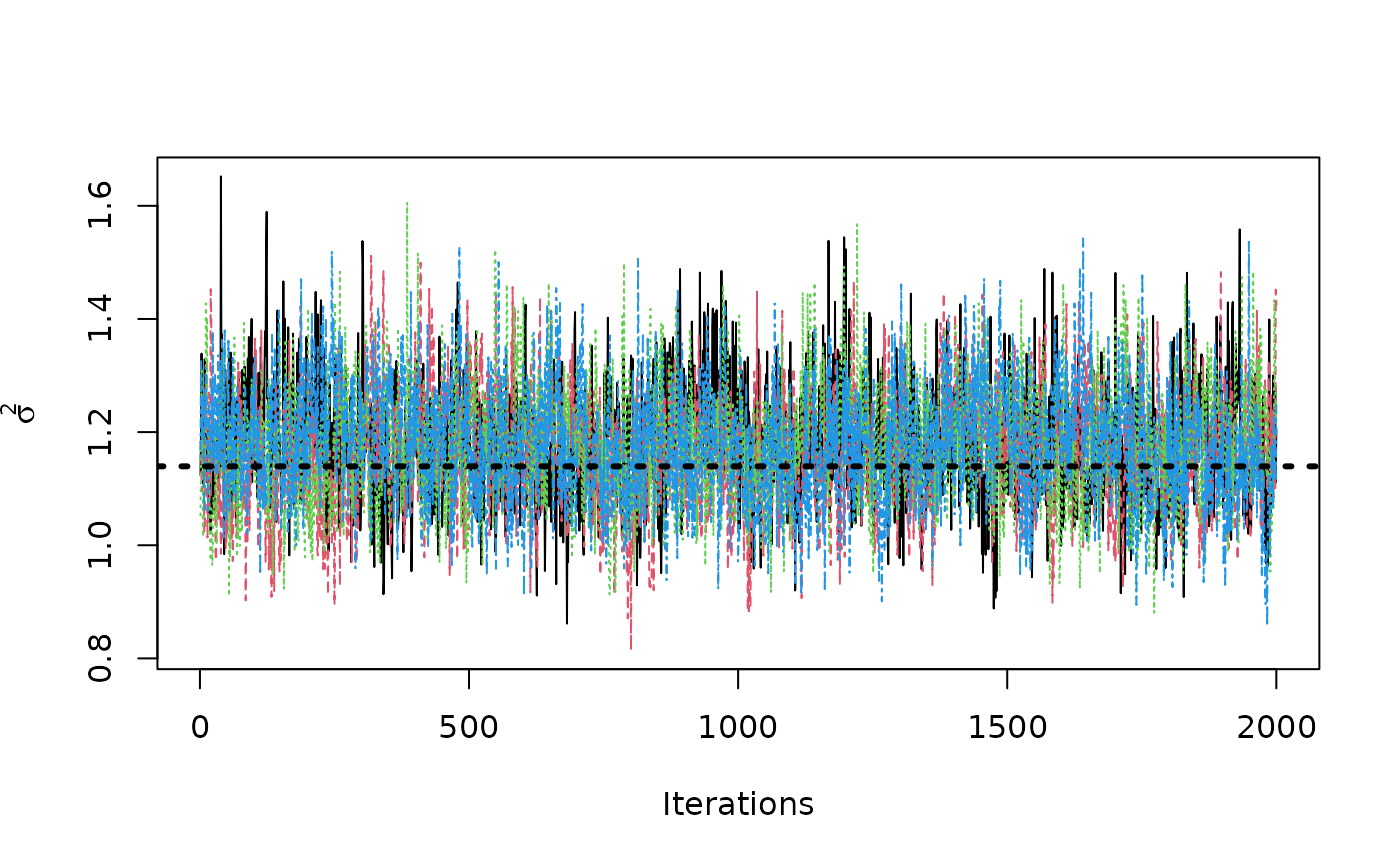
acf <- autocorr.diag(sigma2_coda_list)
ess <- effectiveSize(sigma2_coda_list)
rhat <- gelman.diag(sigma2_coda_list, autoburnin = F)
cat(paste0(
"Average autocorrelation across chains:\n",
paste0(paste0(rownames(acf), ": ", round(acf, 3)), collapse = ", "),
"\nTotal effective sample size across chains: ",
paste0(round(ess, 1), collapse = ", "),
"\n'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): ",
paste0(
round(rhat$psrf[, 1], 3),
collapse = ", "
)
))
#> Average autocorrelation across chains:
#> Lag 0: 1, Lag 1: 0.36, Lag 5: 0.126, Lag 10: 0.079, Lag 50: 0.018
#> Total effective sample size across chains: 1443.4
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): 1.01We can convert this to an array to be consumed by the
bayesplot package
coda_array <- as.array(sigma2_coda_list)
dim(coda_array) <- c(nrow(coda_array), ncol(coda_array), 1)
dimnames(coda_array) <- list(
Iteration = paste0("iter", 1:num_mcmc),
Chain = paste0("chain", 1:num_chains),
Parameter = "sigma2_global"
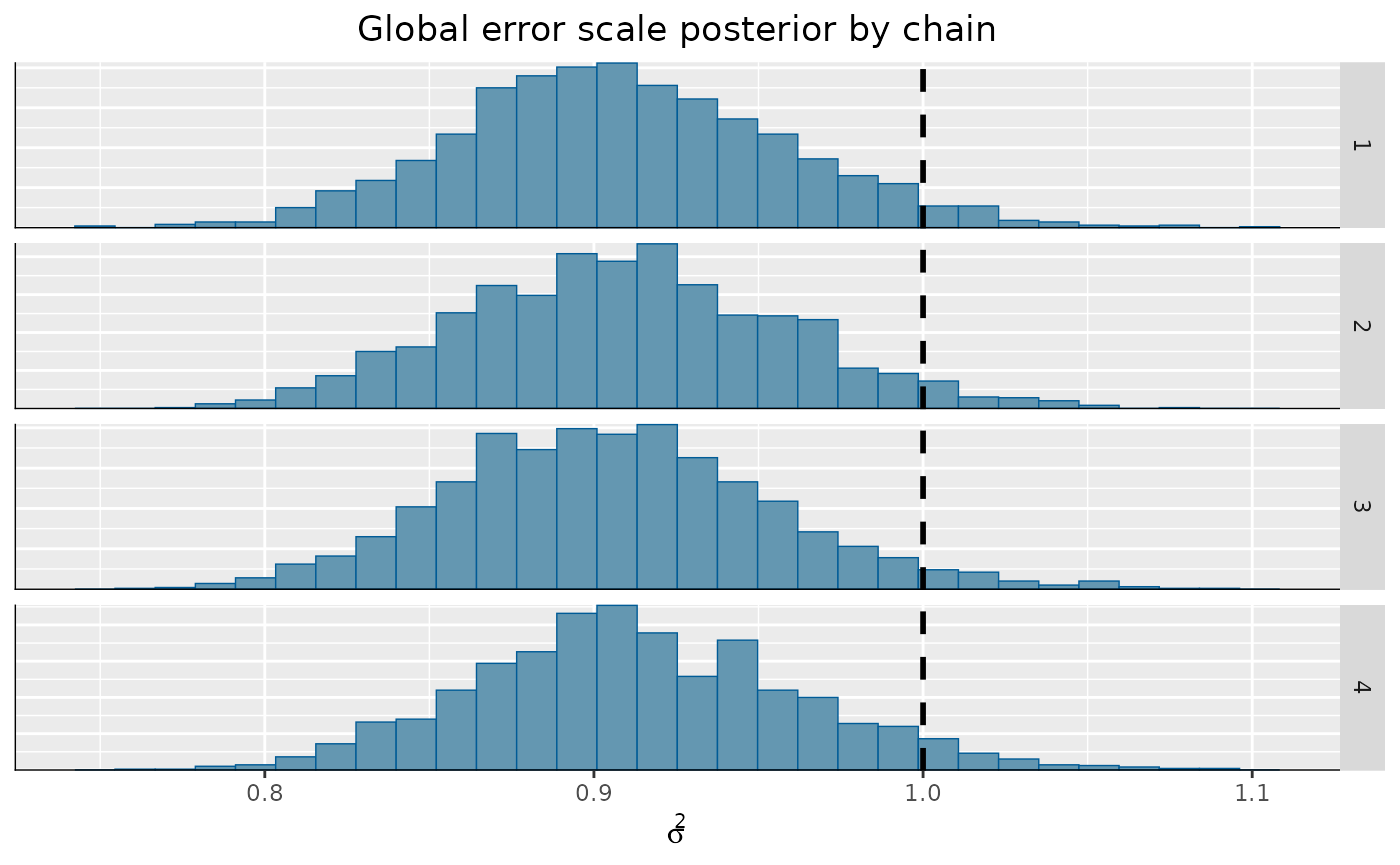
)From here, we can visualize the posterior of for each chain, comparing to the true simulated value.
bayesplot::mcmc_hist_by_chain(
coda_array,
pars = "sigma2_global"
) +
ggplot2::labs(
title = "Global error scale posterior by chain",
x = expression(sigma^2)
) +
ggplot2::theme(
plot.title = ggplot2::element_text(hjust = 0.5)
) +
ggplot2::geom_vline(
xintercept = noise_sd^2,
color = "black",
linetype = "dashed",
size = 1
)
We can also analyze the pointwise forest predictions. Let’s consider 5 observations with the largest test-set residual.
abs_test_set_resid <- abs(y_test - y_hat_test)
top5_resids <- order(abs_test_set_resid, decreasing = T)[1:5]
y_hat_test_posterior <- predict(
bart_model,
X = X_test[top5_resids, ],
leaf_basis = leaf_basis_test[top5_resids],
type = "posterior",
terms = "y_hat"
)
y_hat_coda_list <- coda::as.mcmc.list(lapply(1:num_chains, function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(t(y_hat_test_posterior[, inds_start:inds_end]))
}))
acf <- autocorr.diag(y_hat_coda_list)
ess <- effectiveSize(y_hat_coda_list)
rhat <- gelman.diag(y_hat_coda_list)
cat(
"Average autocorrelation across chains for each of five observations:\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
":\n",
paste0(rownames(acf), ": ", round(acf[, i], 3), collapse = ", "),
"\n"
)
})
),
"\n",
sep = ""
)
#> Average autocorrelation across chains for each of five observations:
#> Observation 41:
#> Lag 0: 1, Lag 1: 0.621, Lag 5: 0.39, Lag 10: 0.299, Lag 50: 0.194
#> Observation 82:
#> Lag 0: 1, Lag 1: 0.508, Lag 5: 0.198, Lag 10: 0.089, Lag 50: 0.043
#> Observation 32:
#> Lag 0: 1, Lag 1: 0.428, Lag 5: 0.172, Lag 10: 0.126, Lag 50: 0.02
#> Observation 93:
#> Lag 0: 1, Lag 1: 0.512, Lag 5: 0.237, Lag 10: 0.181, Lag 50: 0.065
#> Observation 63:
#> Lag 0: 1, Lag 1: 0.577, Lag 5: 0.253, Lag 10: 0.178, Lag 50: 0.069
cat(
"Total effective sample size across chains:\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
": ",
paste0(round(ess[i], 1), collapse = ", "),
"\n"
)
})
),
"\n",
sep = ""
)
#> Total effective sample size across chains:
#> Observation 41: 310.9
#> Observation 82: 1285.8
#> Observation 32: 1258.1
#> Observation 93: 713.6
#> Observation 63: 855.2
cat(
"'R-hat' potential scale reduction factor of Gelman and Rubin (1992)\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
": ",
paste0(round(rhat$psrf[i, 1], 3), collapse = ", "),
"\n"
)
})
),
sep = ""
)
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)
#> Observation 41: 1.093
#> Observation 82: 1.002
#> Observation 32: 1.002
#> Observation 93: 1.025
#> Observation 63: 1.009We can see that these “hard to predict” observations have a higher autocorrelation and lower effective sample size.
Sampling Multiple Chains Sequentially from XBART forests
In the example above, each chain was initialized from “root”, meaning each tree in a forest was a single root node and all parameter values were set to a “default” starting point. If we sample a model using a small number of ‘grow-from-root’ iterations, we can use these forests to initialize MCMC chains.
num_chains <- 4
num_gfr <- 5
num_burnin <- 1000
num_mcmc <- 2000Run the initial GFR sampler
xbart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
num_gfr = num_gfr,
num_burnin = 0,
num_mcmc = 0
)
xbart_model_string <- stochtree::saveBARTModelToJsonString(xbart_model)Run the multi-chain BART sampler, with each chain initialized from a different GFR forest
bart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
num_gfr = num_gfr,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = list(num_chains = num_chains),
previous_model_json = xbart_model_string,
previous_model_warmstart_sample_num = num_gfr
)Now we have a bartmodel object with
num_chains * num_mcmc samples stored internally. These
samples are arranged sequentially, with the first num_mcmc
samples corresponding to chain 1, the next num_mcmc samples
to chain 2, etc…
Since each chain is a set of samples of the same model, we can analyze the samples collectively, for example, by looking at out-of-sample predictions.
y_hat_test <- predict(
bart_model,
X = X_test,
leaf_basis = leaf_basis_test,
type = "mean",
terms = "y_hat"
)
plot(y_hat_test, y_test, xlab = "Predicted", ylab = "Actual")
abline(0, 1, col = "red", lty = 3, lwd = 3)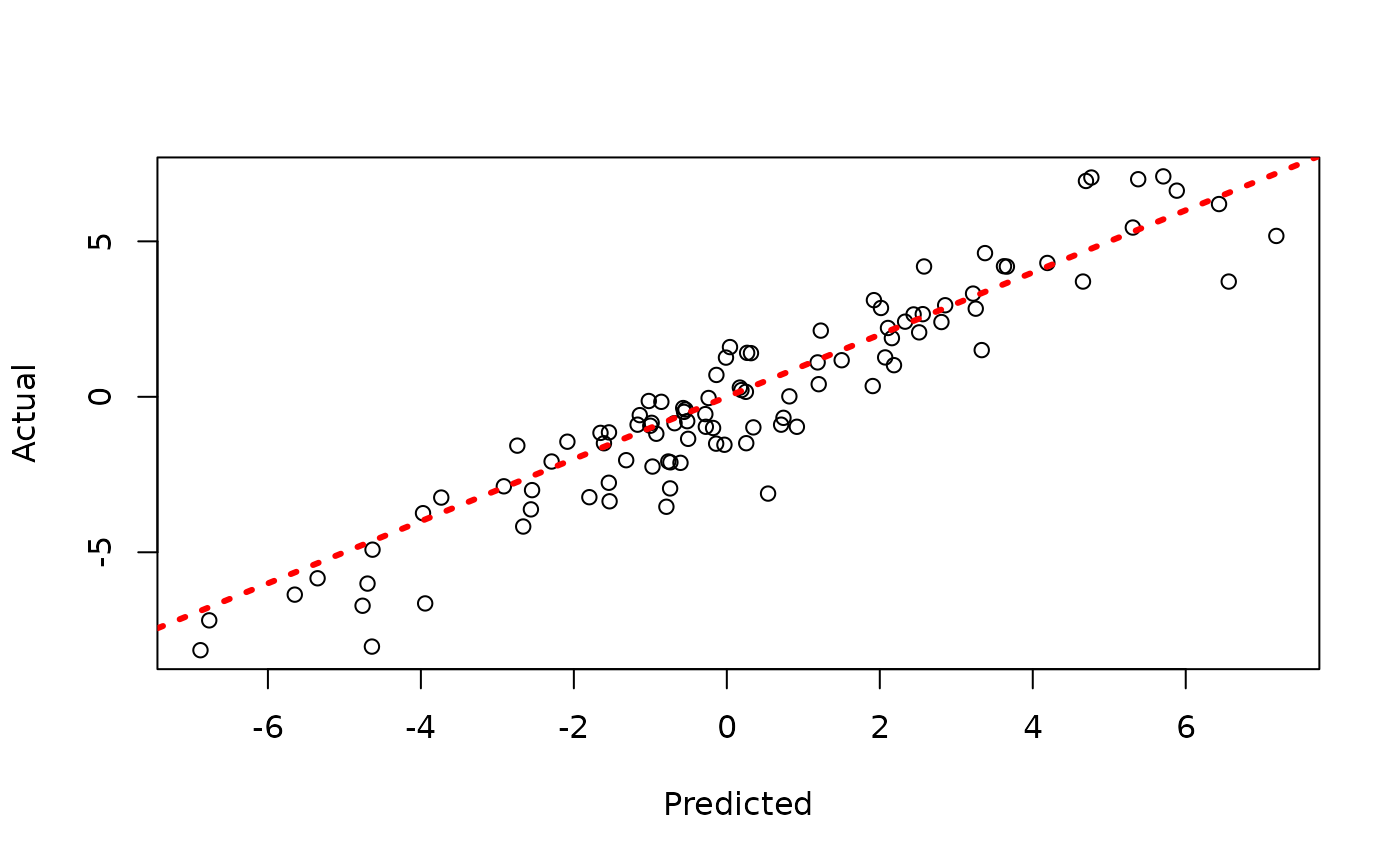
Now, suppose we want to analyze each of the chains separately to assess mixing / convergence.
We can use our knowledge of the internal arrangement of the chain
samples to construct a an mcmc.list in the
coda package, from which we can perform various
diagnostics.
sigma2_coda_list <- coda::as.mcmc.list(lapply(
1:num_chains,
function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(bart_model$sigma2_global_samples[inds_start:inds_end])
}
))
traceplot(sigma2_coda_list, ylab = expression(sigma^2))
abline(h = noise_sd^2, col = "black", lty = 3, lwd = 3)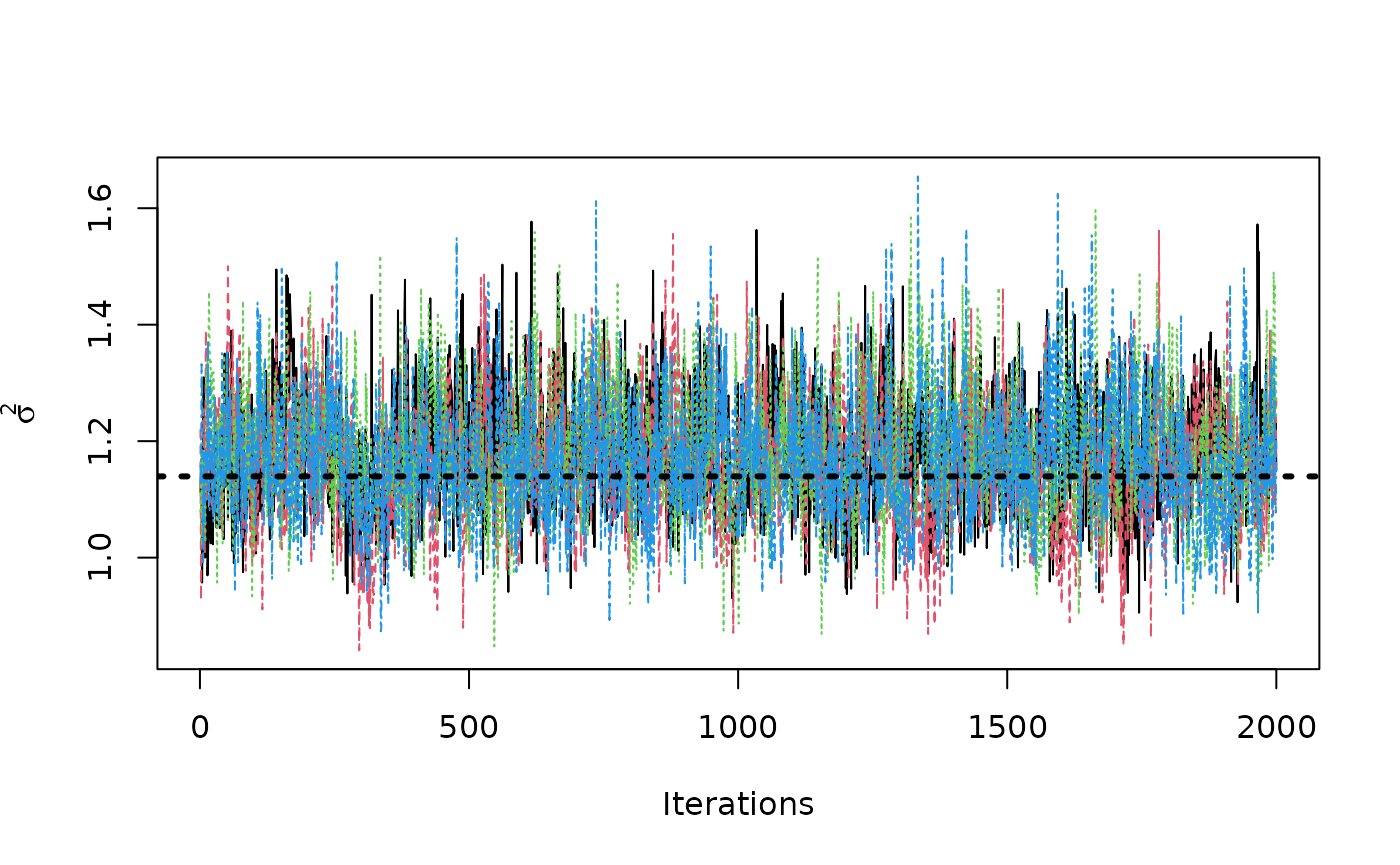
acf <- autocorr.diag(sigma2_coda_list)
ess <- effectiveSize(sigma2_coda_list)
rhat <- gelman.diag(sigma2_coda_list, autoburnin = F)
cat(paste0(
"Average autocorrelation across chains:\n",
paste0(paste0(rownames(acf), ": ", round(acf, 3)), collapse = ", "),
"\nTotal effective sample size across chains: ",
paste0(round(ess, 1), collapse = ", "),
"\n'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): ",
paste0(
round(rhat$psrf[, 1], 3),
collapse = ", "
)
))
#> Average autocorrelation across chains:
#> Lag 0: 1, Lag 1: 0.37, Lag 5: 0.155, Lag 10: 0.13, Lag 50: 0.032
#> Total effective sample size across chains: 1206.4
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): 1.001We can convert this to an array to be consumed by the
bayesplot package
coda_array <- as.array(sigma2_coda_list)
dim(coda_array) <- c(nrow(coda_array), ncol(coda_array), 1)
dimnames(coda_array) <- list(
Iteration = paste0("iter", 1:num_mcmc),
Chain = paste0("chain", 1:num_chains),
Parameter = "sigma2_global"
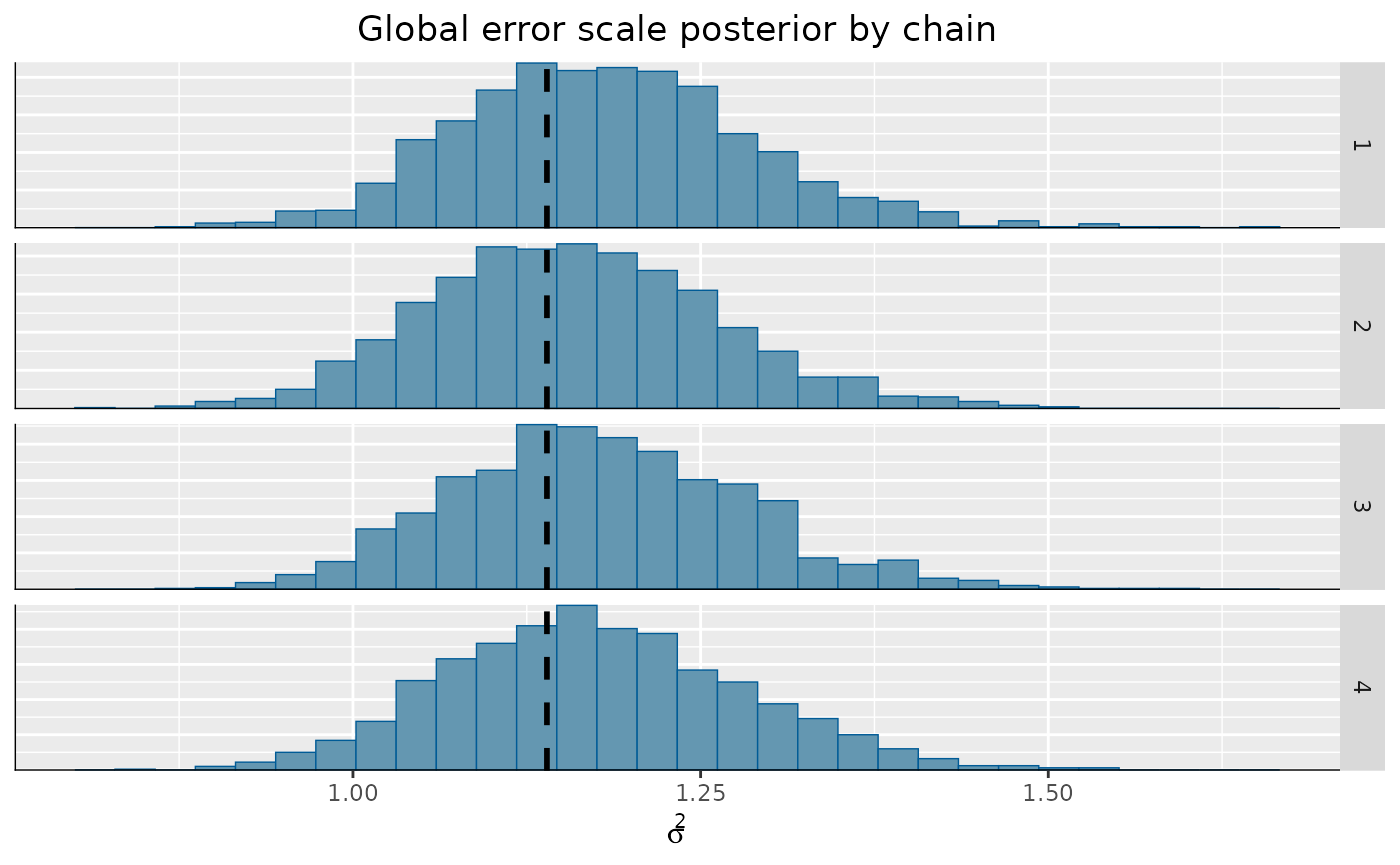
)From here, we can visualize the posterior of for each chain, comparing to the true simulated value.
bayesplot::mcmc_hist_by_chain(
coda_array,
pars = "sigma2_global"
) +
ggplot2::labs(
title = "Global error scale posterior by chain",
x = expression(sigma^2)
) +
ggplot2::theme(
plot.title = ggplot2::element_text(hjust = 0.5)
) +
ggplot2::geom_vline(
xintercept = noise_sd^2,
color = "black",
linetype = "dashed",
size = 1
)
We can also analyze the pointwise forest predictions. Let’s consider 5 observations with the largest test-set residual.
abs_test_set_resid <- abs(y_test - y_hat_test)
top5_resids <- order(abs_test_set_resid, decreasing = T)[1:5]
y_hat_test_posterior <- predict(
bart_model,
X = X_test[top5_resids, ],
leaf_basis = leaf_basis_test[top5_resids],
type = "posterior",
terms = "y_hat"
)
y_hat_coda_list <- coda::as.mcmc.list(lapply(1:num_chains, function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(t(y_hat_test_posterior[, inds_start:inds_end]))
}))
acf <- autocorr.diag(y_hat_coda_list)
ess <- effectiveSize(y_hat_coda_list)
rhat <- gelman.diag(y_hat_coda_list)
cat(
"Average autocorrelation across chains for each of five observations:\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
":\n",
paste0(rownames(acf), ": ", round(acf[, i], 3), collapse = ", "),
"\n"
)
})
),
"\n",
sep = ""
)
#> Average autocorrelation across chains for each of five observations:
#> Observation 41:
#> Lag 0: 1, Lag 1: 0.588, Lag 5: 0.364, Lag 10: 0.285, Lag 50: 0.156
#> Observation 82:
#> Lag 0: 1, Lag 1: 0.528, Lag 5: 0.215, Lag 10: 0.104, Lag 50: 0.028
#> Observation 32:
#> Lag 0: 1, Lag 1: 0.45, Lag 5: 0.218, Lag 10: 0.11, Lag 50: 0.004
#> Observation 63:
#> Lag 0: 1, Lag 1: 0.576, Lag 5: 0.233, Lag 10: 0.145, Lag 50: 0.053
#> Observation 93:
#> Lag 0: 1, Lag 1: 0.519, Lag 5: 0.259, Lag 10: 0.172, Lag 50: 0.032
cat(
"Total effective sample size across chains:\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
": ",
paste0(round(ess[i], 1), collapse = ", "),
"\n"
)
})
),
"\n",
sep = ""
)
#> Total effective sample size across chains:
#> Observation 41: 325.3
#> Observation 82: 1271.9
#> Observation 32: 1419.5
#> Observation 63: 968
#> Observation 93: 859.1
cat(
"'R-hat' potential scale reduction factor of Gelman and Rubin (1992)\n",
paste0(
sapply(1:5, function(i) {
paste0(
"Observation ",
top5_resids[i],
": ",
paste0(round(rhat$psrf[i, 1], 3), collapse = ", "),
"\n"
)
})
),
sep = ""
)
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)
#> Observation 41: 1.04
#> Observation 82: 1.006
#> Observation 32: 1.011
#> Observation 63: 1.013
#> Observation 93: 1.005We can see that some of these “hard to predict” observations have a higher autocorrelation and lower effective sample size.
Sampling Multiple Chains in Parallel
The above example was made somewhat straightforward by the fact that
bart() and bcf() both allow for sequential
multi-chain sampling internally. While we do not currently support
parallel multi-chain sampling internally in the bart() and
bcf() functions, it is possible to do this via
doParallel. While bartmodel or
bcfmodel objects contain external pointers to C++ data
structures (i.e. decision tree ensembles) which are not reachable by
other processes, we can serialize stochtree models to JSON
for cross-process communication. After num_chains models
have been run in parallel and their JSON representations have been
collated in the primary R session, we can combine these into a single
bartmodel or bcfmodel object via the
createBARTModelFromCombinedJsonString() or
createBCFModelFromCombinedJsonString() functions.
In order to run multiple parallel stochtree chains, a parallel backend must be registered in your R environment. The code below will register a parallel backend with access to as many cores are available on your machine. Note that we do not evaluate the code snippet below in order to interact nicely with CRAN / Github Actions environments.
ncores <- parallel::detectCores()
cl <- makeCluster(ncores)
registerDoParallel(cl)Note that the bartmodel object contains external
pointers to forests created by the stochtree shared object,
and when stochtree::bart() is run in parallel on
independent subprocesses, these pointers are not generally accessible in
the session that kicked off the parallel run.
To overcome this, you can return a JSON representation of a
bartmodel in memory and combine them into a single
in-memory bartmodel object.
The first step of this process is to run the sampler in parallel, storing the resulting BART JSON strings in a list.
num_chains <- 4
num_gfr <- 0
num_burnin <- 100
num_mcmc <- 100
bart_model_outputs <- foreach(i = 1:num_chains) %dopar%
{
random_seed <- i
general_params <- list(sample_sigma2_global = T, random_seed = random_seed)
mean_forest_params <- list(sample_sigma2_leaf = F)
bart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
X_test = X_test,
leaf_basis_test = leaf_basis_test,
num_gfr = num_gfr,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = general_params,
mean_forest_params = mean_forest_params
)
bart_model_string <- stochtree::saveBARTModelToJsonString(bart_model)
y_hat_test <- bart_model$y_hat_test
list(model = bart_model_string, yhat = y_hat_test)
}
#> Warning: executing %dopar% sequentially: no parallel backend registeredClose the parallel cluster (not evaluated here, as explained above).
stopCluster(cl)Now, if we want to combine the forests from each of these BART models into a single forest, we can do so as follows
bart_model_strings <- list()
bart_model_yhats <- matrix(NA, nrow = length(y_test), ncol = num_chains)
for (i in 1:length(bart_model_outputs)) {
bart_model_strings[[i]] <- bart_model_outputs[[i]]$model
bart_model_yhats[, i] <- rowMeans(bart_model_outputs[[i]]$yhat)
}
combined_bart <- createBARTModelFromCombinedJsonString(bart_model_strings)We can predict from this combined forest as follows
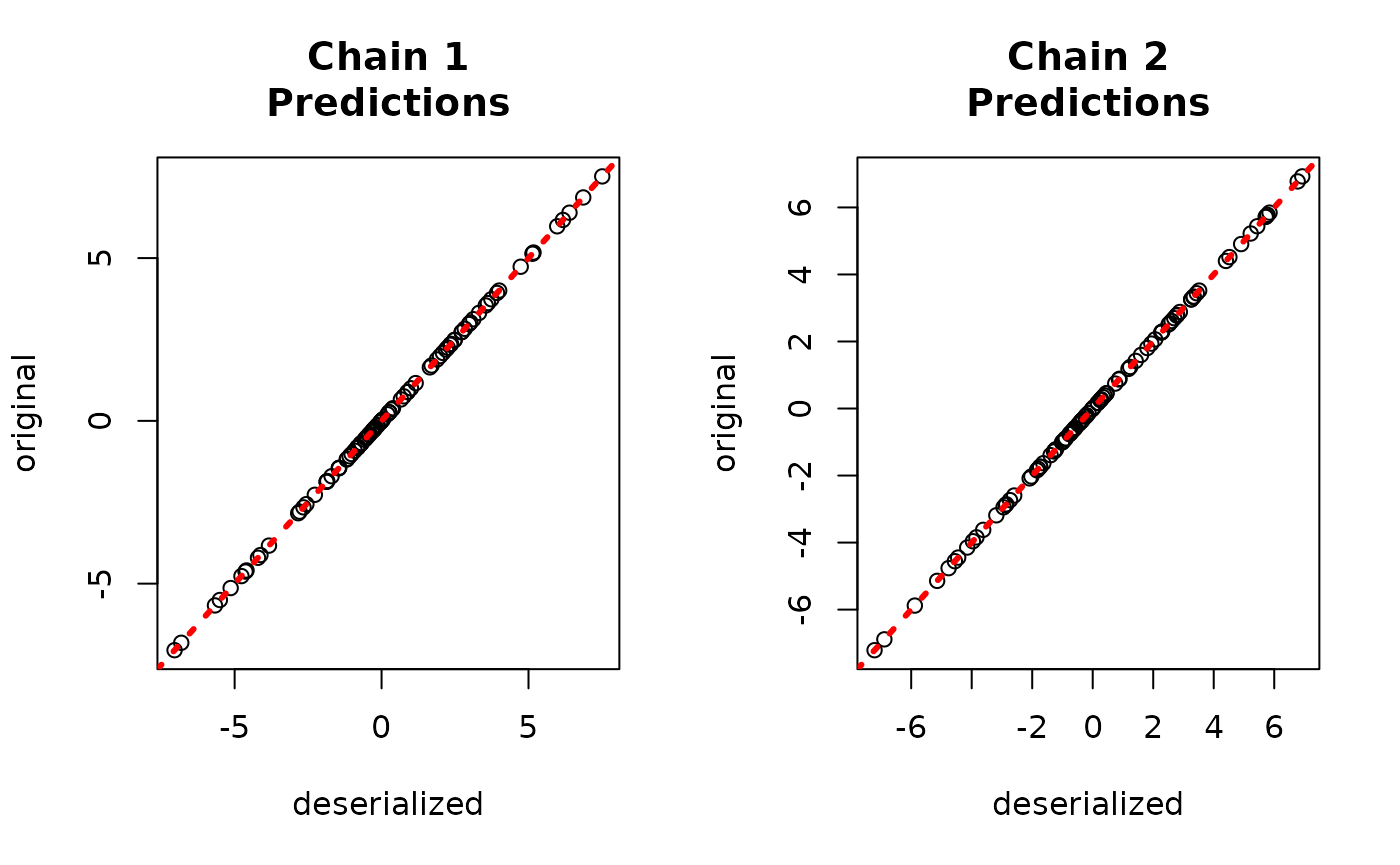
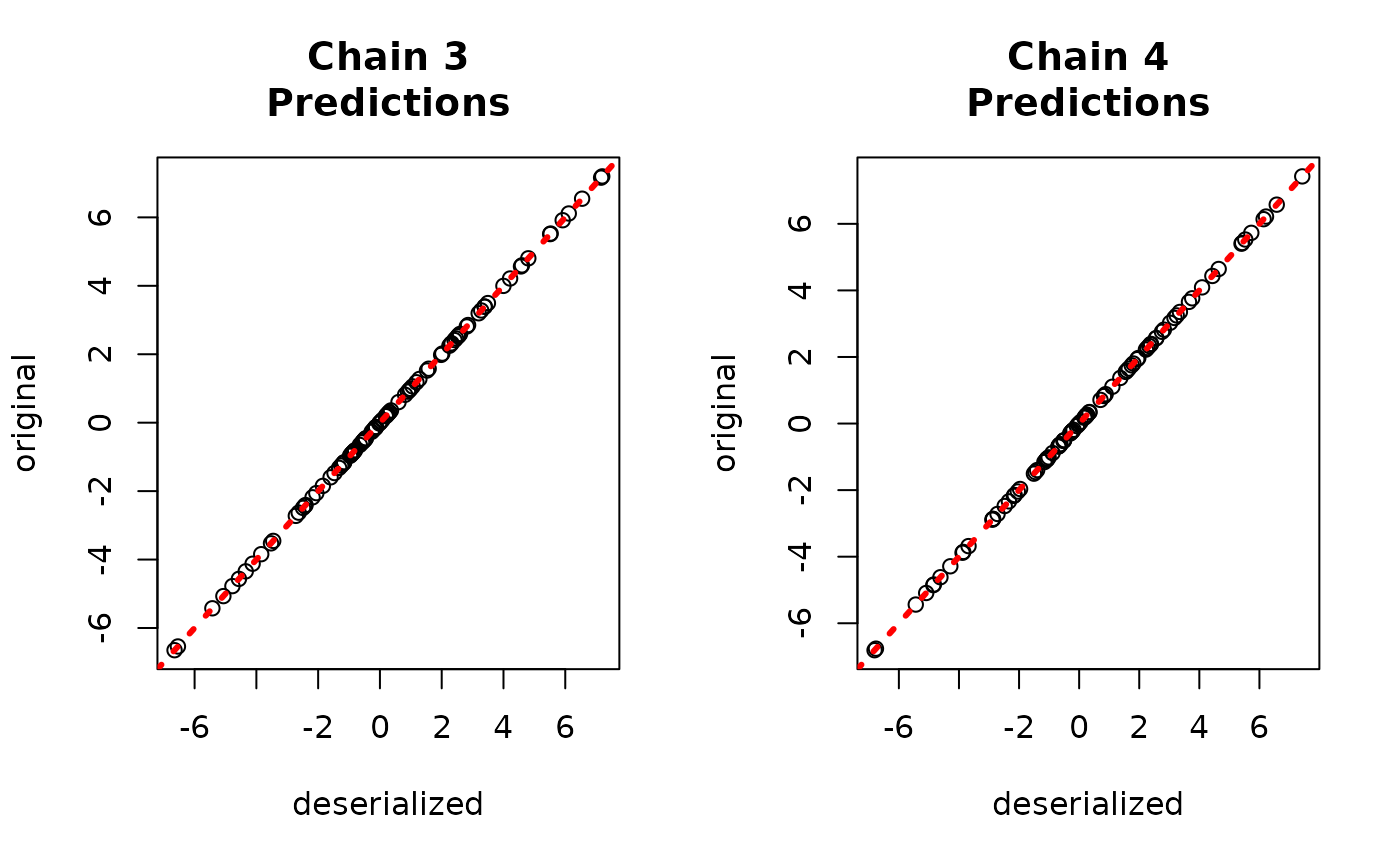
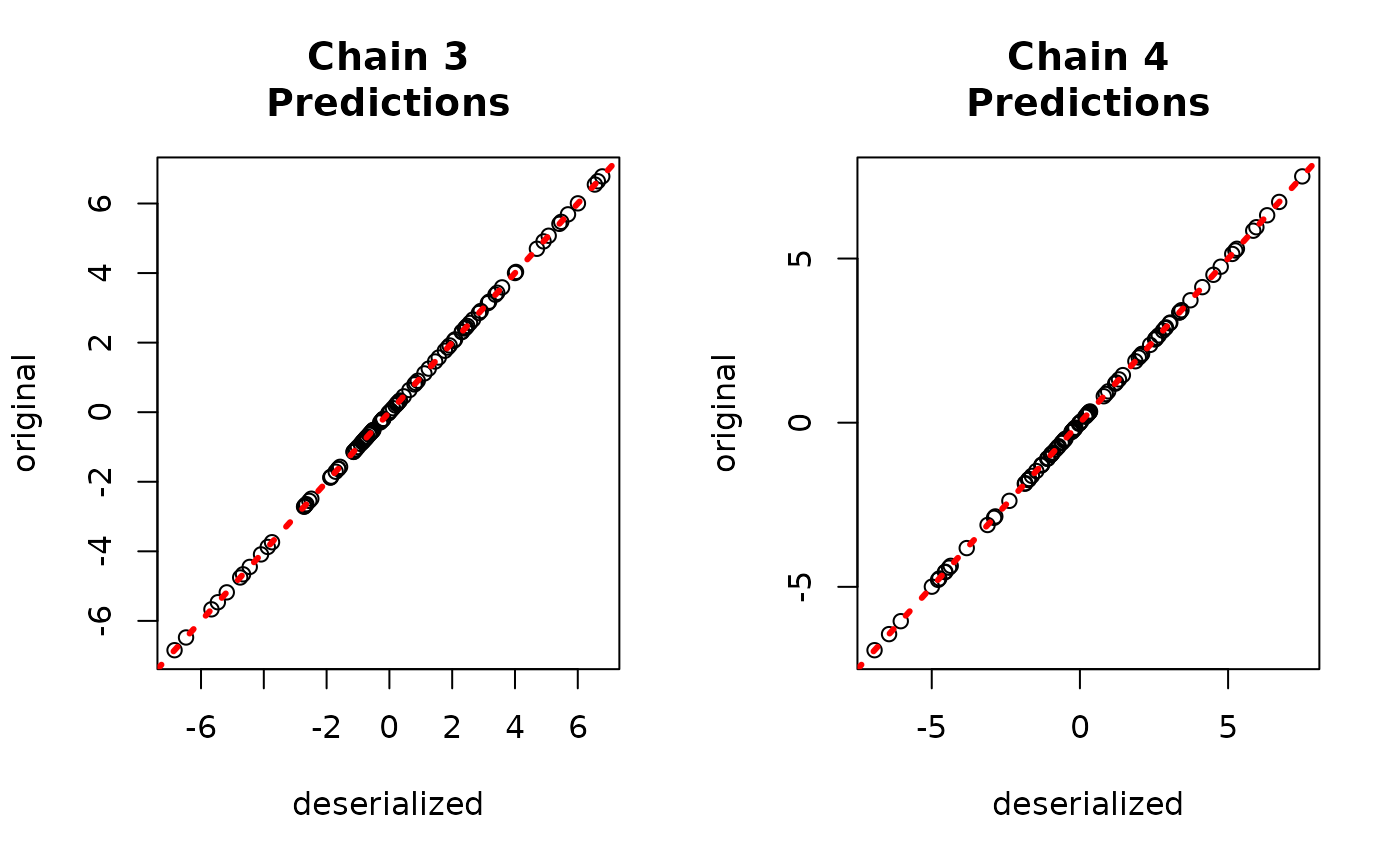
yhat_combined <- predict(combined_bart, X_test, leaf_basis_test)$y_hatCompare average predictions from each chain to the original predictions.
par(mfrow = c(1, 2))
for (i in 1:num_chains) {
offset <- (i - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
plot(
rowMeans(yhat_combined[, inds_start:inds_end]),
bart_model_yhats[, i],
xlab = "deserialized",
ylab = "original",
main = paste0("Chain ", i, "\nPredictions")
)
abline(0, 1, col = "red", lty = 3, lwd = 3)
}
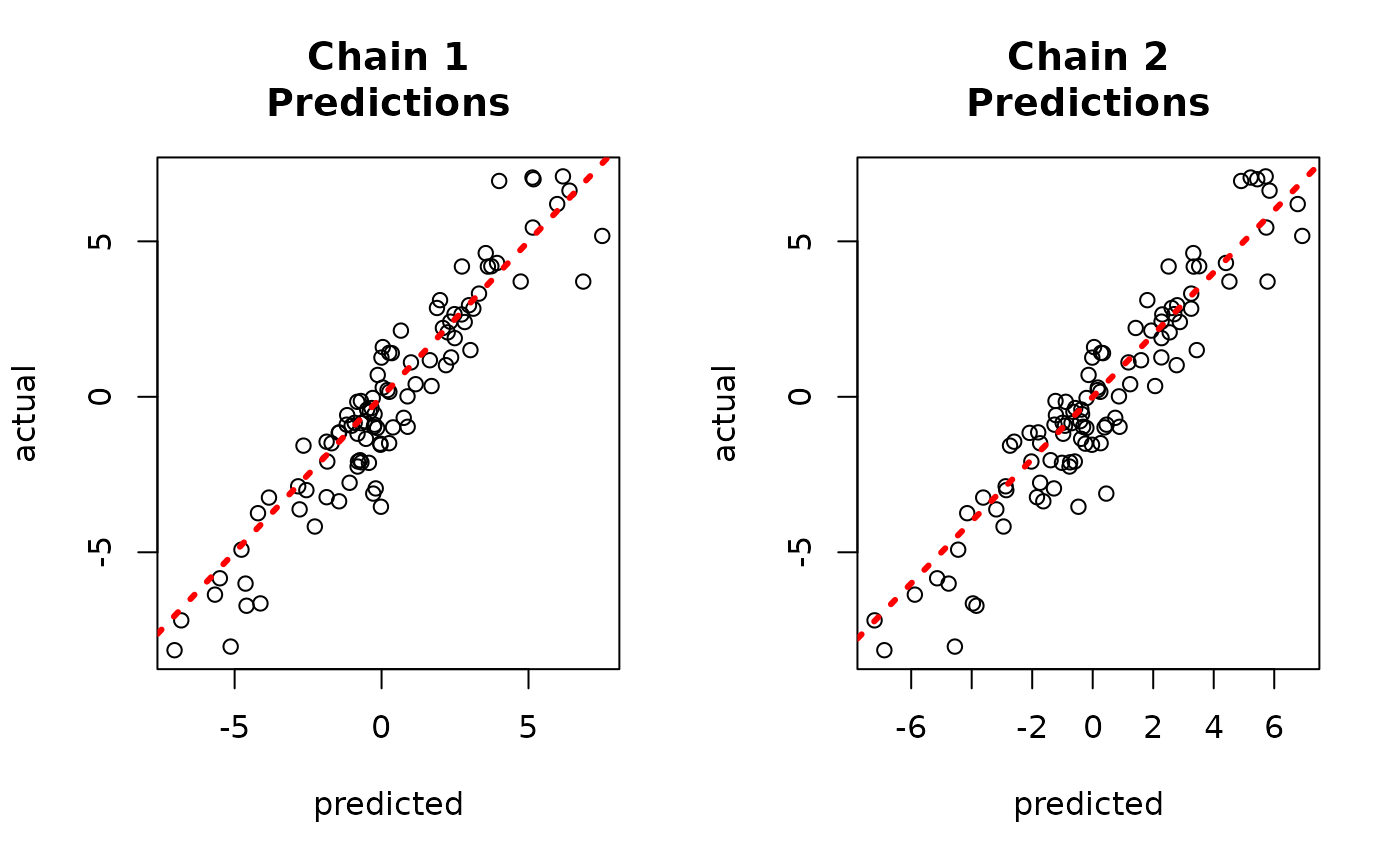
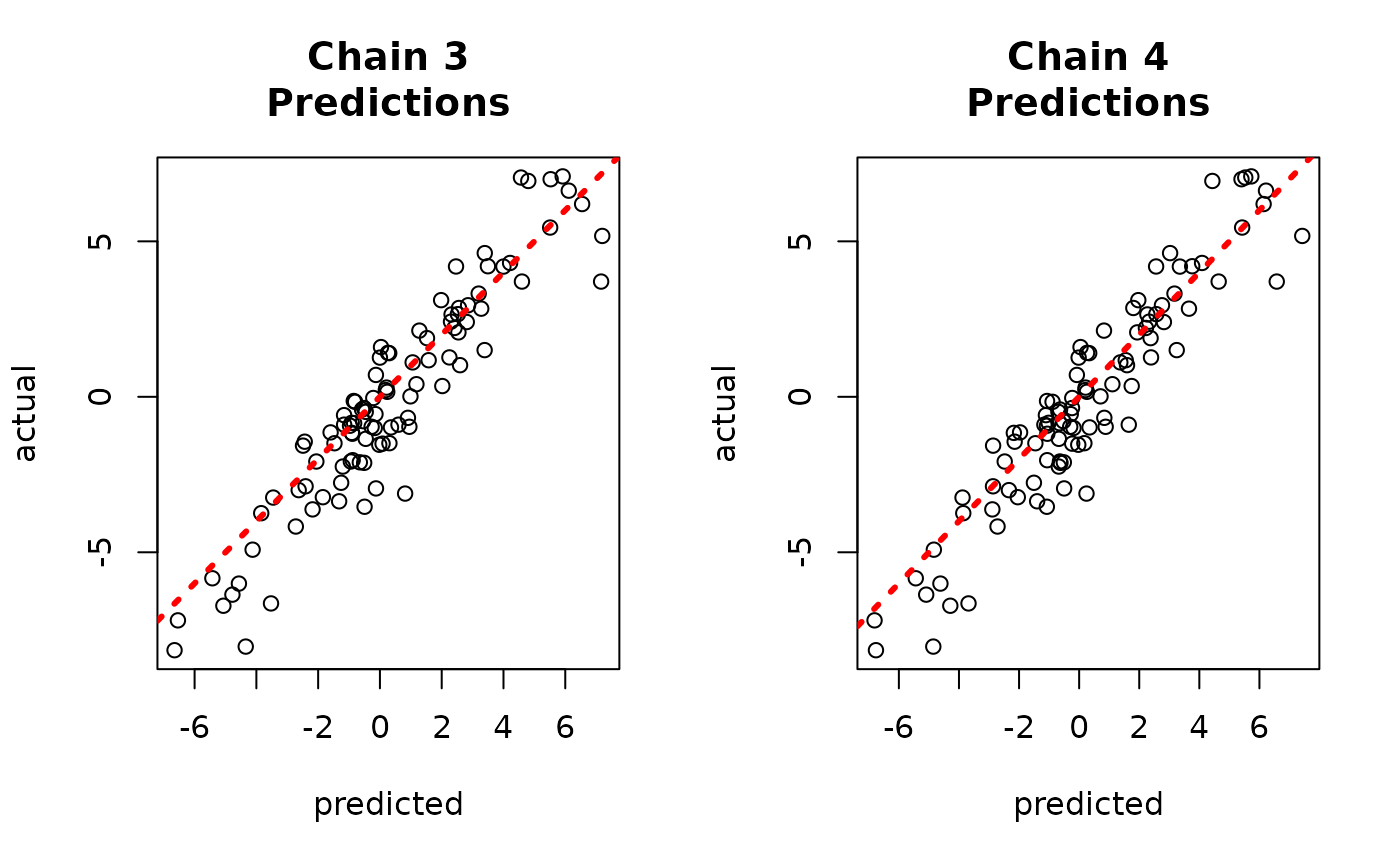
And to the true values.
par(mfrow = c(1, 2))
for (i in 1:num_chains) {
offset <- (i - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
plot(
rowMeans(yhat_combined[, inds_start:inds_end]),
y_test,
xlab = "predicted",
ylab = "actual",
main = paste0("Chain ", i, "\nPredictions")
)
abline(0, 1, col = "red", lty = 3, lwd = 3)
}
Warmstarting Multiple Chains in Parallel
In the above example, we ran multiple parallel chains with each MCMC sampler starting from a “root” forest. Consider instead the “warmstart” approach of He and Hahn (2023), where forests are sampled using the fast “grow-from-root” (GFR) algorithm and then several MCMC chains are run using different GFR forests.
First, we sample this model using the grow-from-root algorithm in the main R session for several iterations (we will use these forests to see independent parallel chains in a moment).
num_chains <- 4
num_gfr <- 5
num_burnin <- 100
num_mcmc <- 100
general_params <- list(sample_sigma2_global = T)
mean_forest_params <- list(sample_sigma2_leaf = T)
xbart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
X_test = X_test,
leaf_basis_test = leaf_basis_test,
num_gfr = num_gfr,
num_burnin = 0,
num_mcmc = 0,
general_params = general_params,
mean_forest_params = mean_forest_params
)
xbart_model_string <- stochtree::saveBARTModelToJsonString(xbart_model)In order to run this sampler in parallel, a parallel backend must be registered in your R environment. The code below will register a parallel backend with access to as many cores are available on your machine. Note that we do not evaluate the code snippet below in order to interact nicely with CRAN / Github Actions environments.
ncores <- parallel::detectCores()
cl <- makeCluster(ncores)
registerDoParallel(cl)Note that the bartmodel object contains external
pointers to forests created by the stochtree shared object,
and when stochtree::bart() is run in parallel on
independent subprocesses, these pointers are not generally accessible in
the session that kicked off the parallel run.
To overcome this, you can return a JSON representation of a
bartmodel in memory and combine them into a single
in-memory bartmodel object.
The first step of this process is to run the sampler in parallel, storing the resulting BART JSON strings in a list.
bart_model_outputs <- foreach(i = 1:num_chains) %dopar%
{
random_seed <- i
general_params <- list(sample_sigma2_global = T, random_seed = random_seed)
mean_forest_params <- list(sample_sigma2_leaf = T)
bart_model <- stochtree::bart(
X_train = X_train,
leaf_basis_train = leaf_basis_train,
y_train = y_train,
X_test = X_test,
leaf_basis_test = leaf_basis_test,
num_gfr = 0,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = general_params,
mean_forest_params = mean_forest_params,
previous_model_json = xbart_model_string,
previous_model_warmstart_sample_num = num_gfr - i + 1,
)
bart_model_string <- stochtree::saveBARTModelToJsonString(bart_model)
y_hat_test <- bart_model$y_hat_test
list(model = bart_model_string, yhat = y_hat_test)
}Close the parallel cluster (not evaluated here, as explained above).
stopCluster(cl)Now, if we want to combine the forests from each of these BART models into a single forest, we can do so as follows
bart_model_strings <- list()
bart_model_yhats <- matrix(NA, nrow = length(y_test), ncol = num_chains)
for (i in 1:length(bart_model_outputs)) {
bart_model_strings[[i]] <- bart_model_outputs[[i]]$model
bart_model_yhats[, i] <- rowMeans(bart_model_outputs[[i]]$yhat)
}
combined_bart <- createBARTModelFromCombinedJsonString(bart_model_strings)We can predict from this combined forest as follows
yhat_combined <- predict(combined_bart, X_test, leaf_basis_test)$y_hatCompare average predictions from each chain to the original predictions.
par(mfrow = c(1, 2))
for (i in 1:num_chains) {
offset <- (i - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
plot(
rowMeans(yhat_combined[, inds_start:inds_end]),
bart_model_yhats[, i],
xlab = "deserialized",
ylab = "original",
main = paste0("Chain ", i, "\nPredictions")
)
abline(0, 1, col = "red", lty = 3, lwd = 3)
}
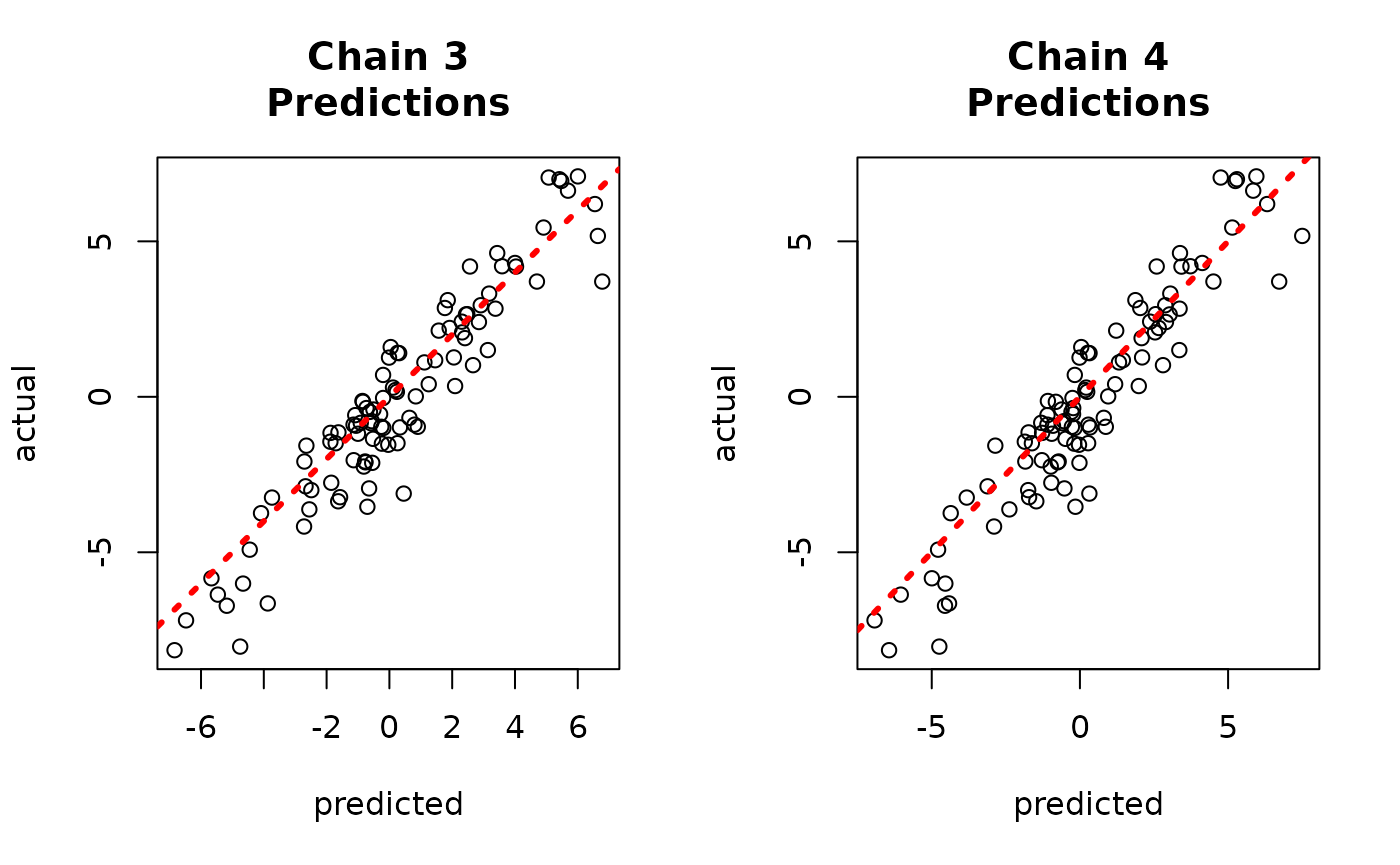
And to the true values.
par(mfrow = c(1, 2))
for (i in 1:num_chains) {
offset <- (i - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
plot(
rowMeans(yhat_combined[, inds_start:inds_end]),
y_test,
xlab = "predicted",
ylab = "actual",
main = paste0("Chain ", i, "\nPredictions")
)
abline(0, 1, col = "red", lty = 3, lwd = 3)
}
Demo 2: Causal Inference
Data Simulation
Simulate a simple causal model
# Generate the data
n <- 1000
p <- 5
X <- matrix(runif(n * p), ncol = p)
pi_x <- 0.25 + 0.5 * X[, 1]
mu_x <- pi_x * 5 + 2 * X[, 3]
tau_x <- X[, 2] * 2 - 1
Z <- rbinom(n, 1, pi_x)
E_Y_XZ <- mu_x + tau_x * Z
y <- E_Y_XZ + rnorm(n, 0, 1)
# Split data into test and train sets
test_set_pct <- 0.2
n_test <- round(test_set_pct * n)
n_train <- n - n_test
test_inds <- sort(sample(1:n, n_test, replace = FALSE))
train_inds <- (1:n)[!((1:n) %in% test_inds)]
X_test <- X[test_inds, ]
X_train <- X[train_inds, ]
Z_test <- Z[test_inds]
Z_train <- Z[train_inds]
propensity_test <- pi_x[test_inds]
propensity_train <- pi_x[train_inds]
y_test <- y[test_inds]
y_train <- y[train_inds]Sampling Multiple Chains Sequentially from Scratch
As with the supervised learning (BART) demo, we run 4 independent chains with 2000 MCMC iterations, each of which is burned in for 1000 iterations.
num_chains <- 4
num_gfr <- 0
num_burnin <- 1000
num_mcmc <- 2000Run the sampler
general_params <- list(
num_chains = num_chains
)
bcf_model <- stochtree::bcf(
X_train = X_train,
Z_train = Z_train,
propensity_train = propensity_train,
y_train = y_train,
num_gfr = num_gfr,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = general_params
)Now we have a bcfmodel object with
num_chains * num_mcmc samples stored internally. These
samples are arranged sequentially, with the first num_mcmc
samples corresponding to chain 1, the next num_mcmc samples
to chain 2, etc…
Since each chain is a set of samples of the same model, we can analyze the samples collectively, for example, by looking at out-of-sample predictions.
y_hat_test <- predict(
bcf_model,
X = X_test,
Z = Z_test,
propensity = propensity_test,
type = "mean",
terms = "y_hat"
)
plot(y_hat_test, y_test, xlab = "Predicted", ylab = "Actual")
abline(0, 1, col = "red", lty = 3, lwd = 3)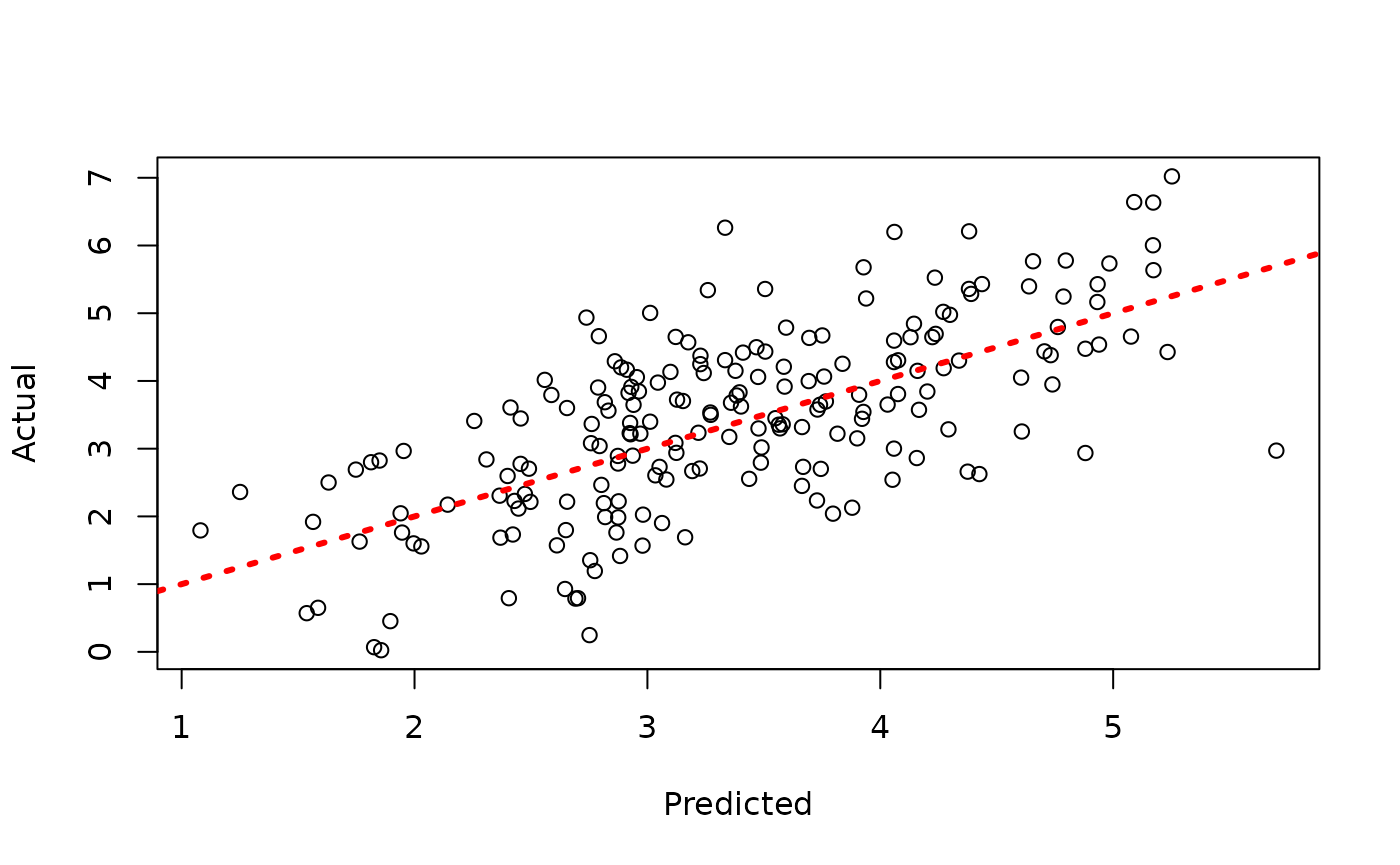
Now, suppose we want to analyze each of the chains separately to assess mixing / convergence.
We can use our knowledge of the internal arrangement of the chain
samples to construct a an mcmc.list in the
coda package, from which we can perform various
diagnostics.
sigma2_coda_list <- coda::as.mcmc.list(lapply(
1:num_chains,
function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(bcf_model$sigma2_global_samples[inds_start:inds_end])
}
))
traceplot(sigma2_coda_list, ylab = expression(sigma^2))
abline(h = 1, col = "black", lty = 3, lwd = 3)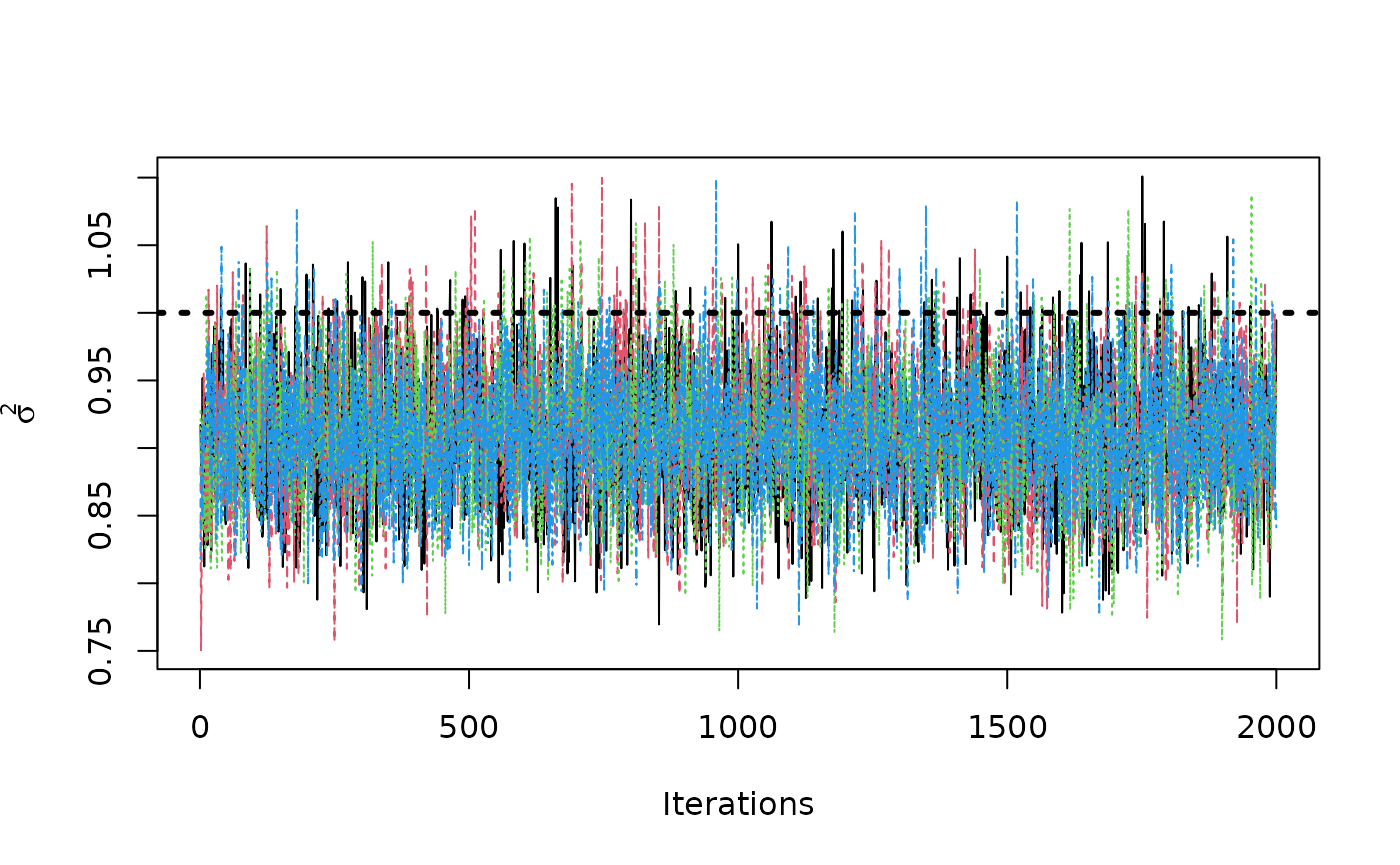
acf <- autocorr.diag(sigma2_coda_list)
ess <- effectiveSize(sigma2_coda_list)
rhat <- gelman.diag(sigma2_coda_list, autoburnin = F)
cat(paste0(
"Average autocorrelation across chains:\n",
paste0(paste0(rownames(acf), ": ", round(acf, 3)), collapse = ", "),
"\nTotal effective sample size across chains: ",
paste0(round(ess, 1), collapse = ", "),
"\n'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): ",
paste0(
round(rhat$psrf[, 1], 3),
collapse = ", "
)
))
#> Average autocorrelation across chains:
#> Lag 0: 1, Lag 1: 0.118, Lag 5: 0.014, Lag 10: 0.018, Lag 50: 0.005
#> Total effective sample size across chains: 5334.7
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): 1We can convert this to an array to be consumed by the
bayesplot package
coda_array <- as.array(sigma2_coda_list)
dim(coda_array) <- c(nrow(coda_array), ncol(coda_array), 1)
dimnames(coda_array) <- list(
Iteration = paste0("iter", 1:num_mcmc),
Chain = paste0("chain", 1:num_chains),
Parameter = "sigma2_global"
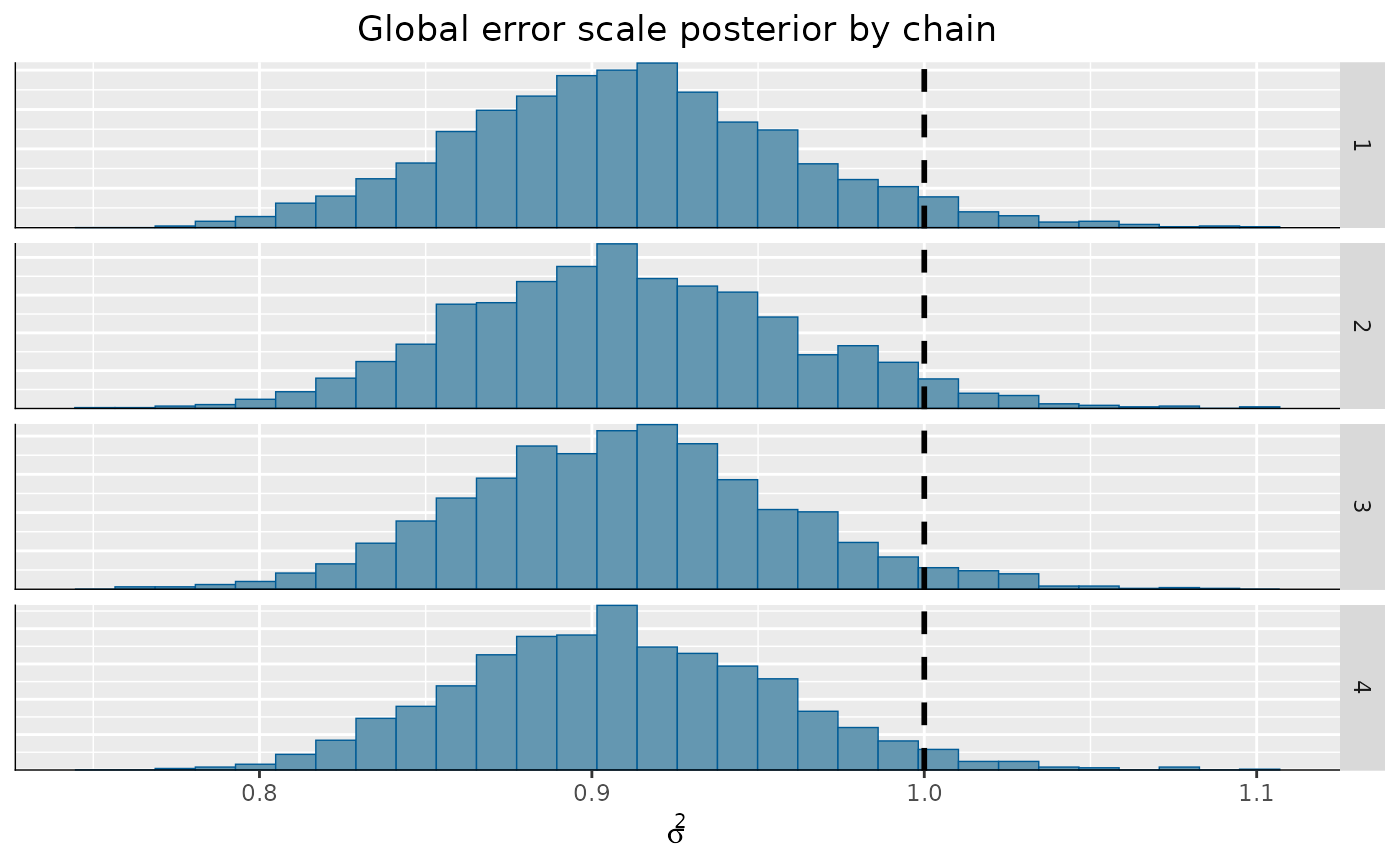
)From here, we can visualize the posterior of for each chain, comparing to the true simulated value.
bayesplot::mcmc_hist_by_chain(
coda_array,
pars = "sigma2_global"
) +
ggplot2::labs(
title = "Global error scale posterior by chain",
x = expression(sigma^2)
) +
ggplot2::theme(
plot.title = ggplot2::element_text(hjust = 0.5)
) +
ggplot2::geom_vline(
xintercept = 1,
color = "black",
linetype = "dashed",
size = 1
)
Sampling Multiple Chains Sequentially from XBCF Forests
As with the second supervised learning (BART) demo, we run 4 independent chains with 2000 MCMC iterations, each of which is burned in for 1000 iterations after being initialized from a different GFR forest.
num_chains <- 4
num_gfr <- 5
num_burnin <- 1000
num_mcmc <- 2000Run the initial GFR sampler
xbcf_model <- stochtree::bcf(
X_train = X_train,
Z_train = Z_train,
propensity_train = propensity_train,
y_train = y_train,
num_gfr = num_gfr,
num_burnin = 0,
num_mcmc = 0
)
xbcf_model_string <- stochtree::saveBCFModelToJsonString(xbcf_model)Run the multi-chain BCF sampler, with each chain initialized from a different GFR forest
general_params <- list(
num_chains = num_chains
)
bcf_model <- stochtree::bcf(
X_train = X_train,
Z_train = Z_train,
propensity_train = propensity_train,
y_train = y_train,
num_gfr = 0,
num_burnin = num_burnin,
num_mcmc = num_mcmc,
general_params = general_params,
previous_model_json = xbcf_model_string,
previous_model_warmstart_sample_num = num_gfr
)Now we have a bcfmodel object with
num_chains * num_mcmc samples stored internally. These
samples are arranged sequentially, with the first num_mcmc
samples corresponding to chain 1, the next num_mcmc samples
to chain 2, etc…
Since each chain is a set of samples of the same model, we can analyze the samples collectively, for example, by looking at out-of-sample predictions.
y_hat_test <- predict(
bcf_model,
X = X_test,
Z = Z_test,
propensity = propensity_test,
type = "mean",
terms = "y_hat"
)
plot(y_hat_test, y_test, xlab = "Predicted", ylab = "Actual")
abline(0, 1, col = "red", lty = 3, lwd = 3)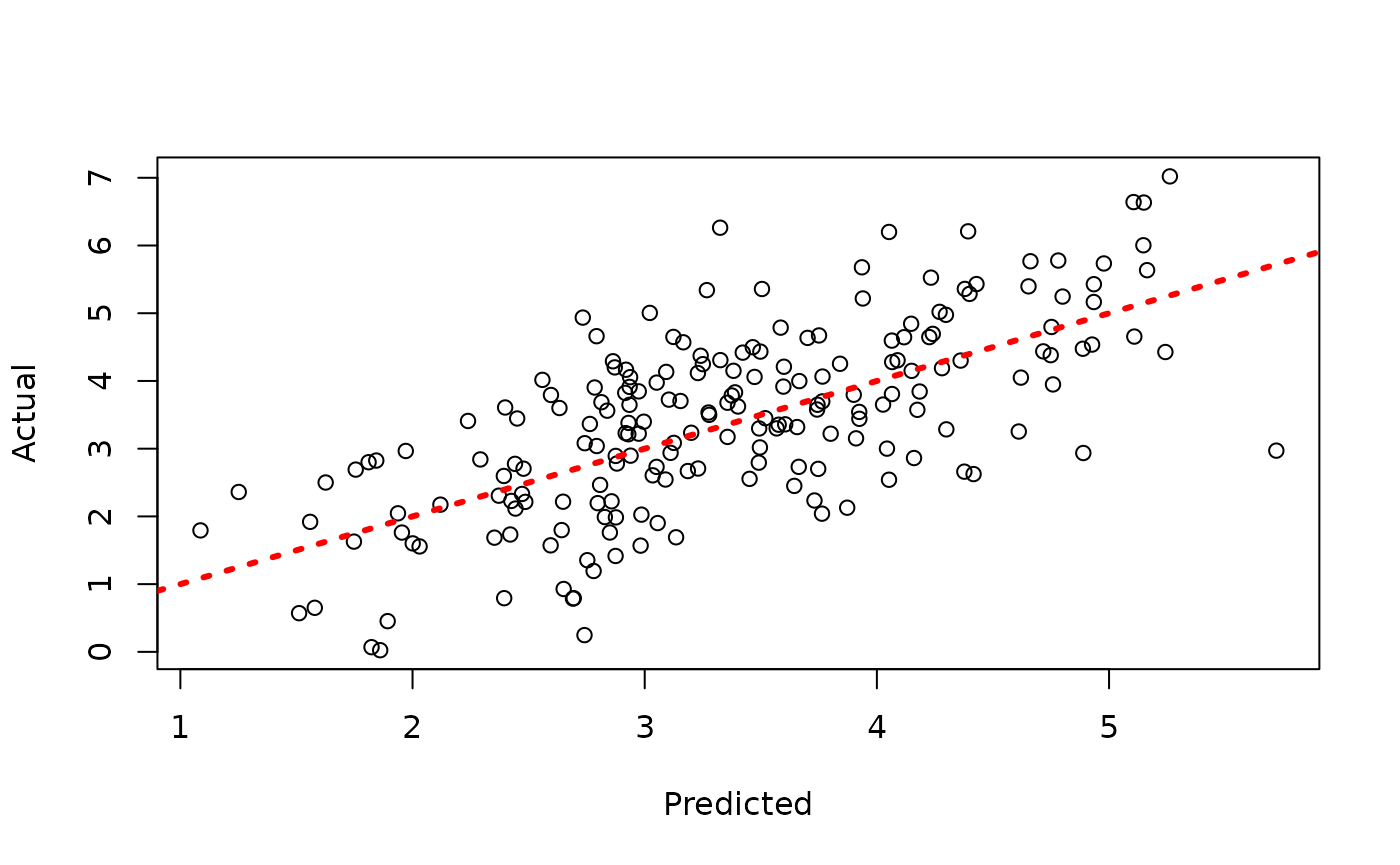
Now, suppose we want to analyze each of the chains separately to assess mixing / convergence.
We can use our knowledge of the internal arrangement of the chain
samples to construct a an mcmc.list in the
coda package, from which we can perform various
diagnostics.
sigma2_coda_list <- coda::as.mcmc.list(lapply(
1:num_chains,
function(chain_idx) {
offset <- (chain_idx - 1) * num_mcmc
inds_start <- offset + 1
inds_end <- offset + num_mcmc
coda::mcmc(bcf_model$sigma2_global_samples[inds_start:inds_end])
}
))
traceplot(sigma2_coda_list, ylab = expression(sigma^2))
abline(h = 1, col = "black", lty = 3, lwd = 3)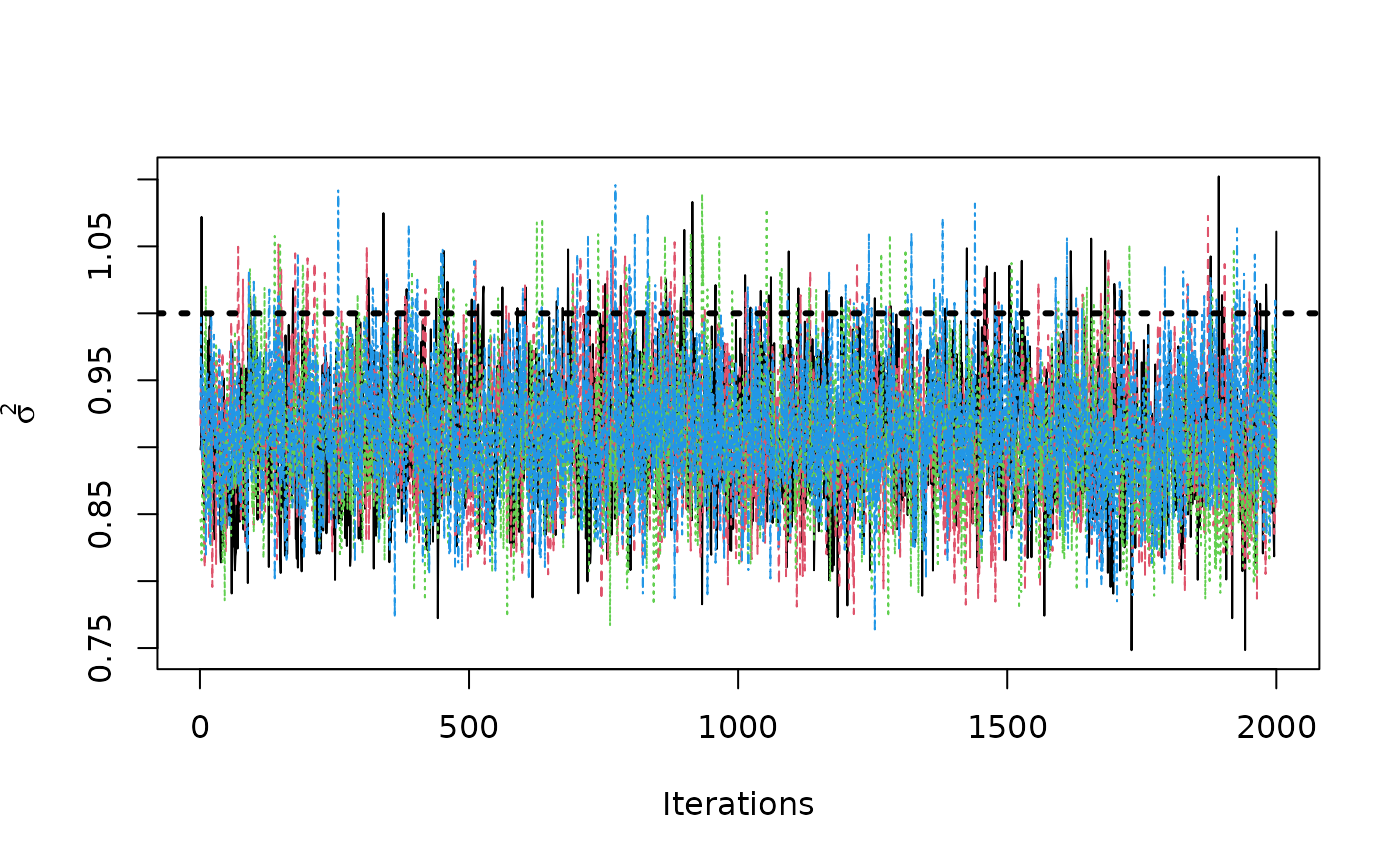
acf <- autocorr.diag(sigma2_coda_list)
ess <- effectiveSize(sigma2_coda_list)
rhat <- gelman.diag(sigma2_coda_list, autoburnin = F)
cat(paste0(
"Average autocorrelation across chains:\n",
paste0(paste0(rownames(acf), ": ", round(acf, 3)), collapse = ", "),
"\nTotal effective sample size across chains: ",
paste0(round(ess, 1), collapse = ", "),
"\n'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): ",
paste0(
round(rhat$psrf[, 1], 3),
collapse = ", "
)
))
#> Average autocorrelation across chains:
#> Lag 0: 1, Lag 1: 0.118, Lag 5: 0.042, Lag 10: 0.024, Lag 50: -0.005
#> Total effective sample size across chains: 5032.4
#> 'R-hat' potential scale reduction factor of Gelman and Rubin (1992)): 1.003We can convert this to an array to be consumed by the
bayesplot package
coda_array <- as.array(sigma2_coda_list)
dim(coda_array) <- c(nrow(coda_array), ncol(coda_array), 1)
dimnames(coda_array) <- list(
Iteration = paste0("iter", 1:num_mcmc),
Chain = paste0("chain", 1:num_chains),
Parameter = "sigma2_global"
)From here, we can visualize the posterior of for each chain, comparing to the true simulated value.
bayesplot::mcmc_hist_by_chain(
coda_array,
pars = "sigma2_global"
) +
ggplot2::labs(
title = "Global error scale posterior by chain",
x = expression(sigma^2)
) +
ggplot2::theme(
plot.title = ggplot2::element_text(hjust = 0.5)
) +
ggplot2::geom_vline(
xintercept = 1,
color = "black",
linetype = "dashed",
size = 1
)